Abstract
Background: In the ELARA (E2202) trial (NCT03568461), tisagenlecleucel (tisa-cel) demonstrated high response rates in patients with relapsed/refractory follicular lymphoma (r/r FL), including those with high-risk disease, with an overall response rate (ORR) of 86% and complete response rate (CRR) of 66%. As ELARA did not include a comparator arm, an adjusted indirect treatment comparison (ITC) using patient-level data from a global retrospective cohort study was conducted. This study aimed to provide comparative, contextual evidence to the efficacy outcomes of tisa-cel from ELARA relative to standard of care (SOC) in routine practice.
Methods: ELARA is an ongoing, single-arm, global, multicenter, phase II trial which evaluates the efficacy and safety of tisa-cel in adult patients with r/r FL. As of March 29, 2021, 98 patients were enrolled with a median follow-up of 15 months. SOC data were obtained from ReCORD-FL, a global retrospective cohort study of clinical outcomes in patients with r/r FL meeting the ELARA eligibility criteria who were treated per routine practice at 10 academic centers across North America and Europe; 7 ReCORD-FL sites are also participating in ELARA, but no patients are enrolled in both studies. In ReCORD-FL, with a data cutoff date of December 31, 2020, a total of 187 patients with ≥2 lines of previous treatment were identified for inclusion, with a median follow-up from third-line of 57 months. A complete-case comparison analysis was performed for 97 ELARA apheresed patients and 143 ReCORD-FL patients with complete data on all baseline variables and prognostic factors used for adjustment. For the comparative analysis, one line of therapy (LoT) per patient was selected using a propensity score model to identify the LoT which had the highest chance of being enrolled in ELARA conditional on baseline characteristics and prognostic factors available at the start of the LoT (i.e., the selected LoT was the one with the highest propensity score to be in ELARA). After selection of LoT, an adjusted ITC was performed to assess the effect of tisa-cel versus SOC by measuring CRR, ORR, progression-free survival (PFS), overall survival (OS), and time to next treatment (TTNT). A subgroup analysis of SOC patients with ≥1 eligible LoT initiated from 2014 on (coinciding with the introduction of the Lugano response criteria as well as regulatory approval of idelalisib) was performed for all endpoints.
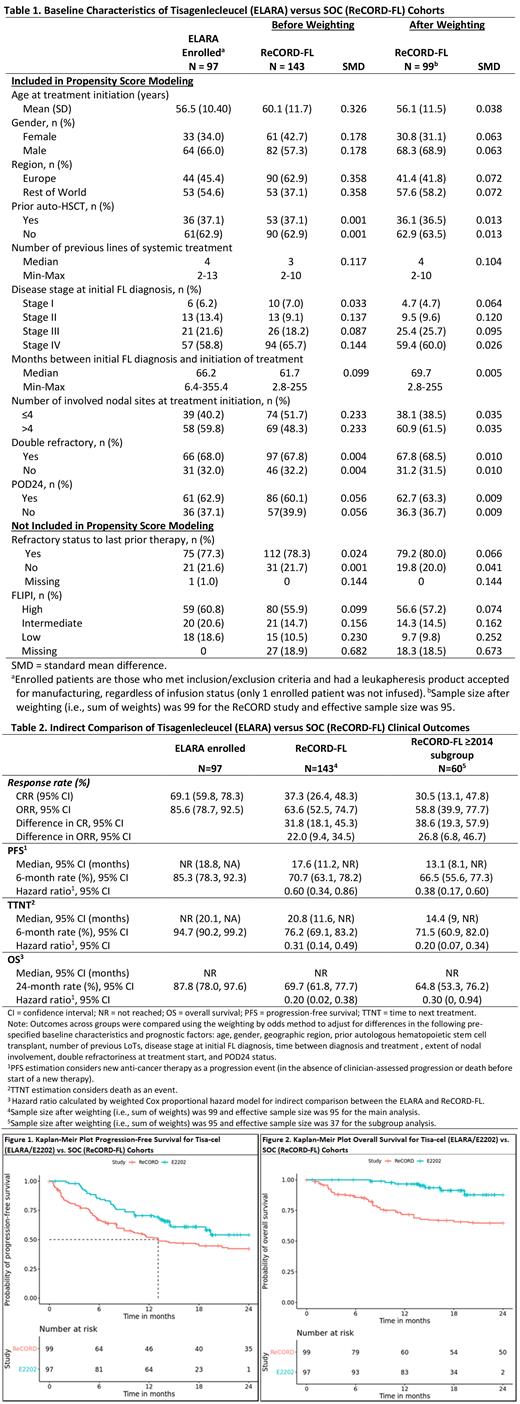
Results: Baseline characteristics for the tisa-cel and SOC cohorts are described in Table 1; after weighting, baseline variables, including number of previous lines of systemic therapy (median: 4 lines) were well balanced between the tisa-cel and SOC cohorts. Treatment regimens observed for ReCORD-FL patients at LoT selection were: anti-CD20 antibody plus alkylator (31.5% of patients), anti-CD20 antibody without alkylator (25.9%), alkylator without anti-CD20 antibody (17.5%), and regimens other than anti-CD20 antibody and alkylator (25.2%). After LoT selection and adjusting for differences in baseline variables, tisa-cel was associated with improvement over SOC in CRR (69.1% vs. 37.3%), ORR (85.6% vs. 63.6%), as well as PFS, TTNT and OS (Table 2, Figures 1-2), with a numerically higher 6-month PFS rate vs. SOC (85.3% vs. 66.5%), as well as higher 24-month OS rate (87.8% vs. 64.8%). Further, there was an estimated 80% reduction in death risk in favor of tisa-cel over SOC, a 40% reduction in risk of progression in favor of tisa-cel over SOC and a 69% reduction in risk of death or requiring a new anticancer therapy (Table 1). In the sub-analysis of SOC patients with lines of therapy initiated in or after 2014, the superiority of tisa-cel over SOC was confirmed in all the efficacy outcomes (CRR: 69.1% vs. 30.5%; ORR: 85.6% vs. 58.8%; hazard ratios substantially < 1 for OS, PFS, TTNT).
Conclusion: The ITC results suggest that tisa-cel has superior efficacy over SOC in r/r FL for all evaluated endpoints. A key limitation of this study is that response assessment criteria and schedule was more heterogeneous in ReCORD-FL than in ELARA. However, the sub-analysis on SOC patients assessed with a LoT selected in 2014 or later (when more patients could have been assessed using the Lugano response criteria) showed similar favorability for tisa-cel over SOC in all the efficacy outcomes. Moreover, outcome parameters independent of response criteria, namely OS and TTNT, were also significantly better for tisa-cel vs. SOC.
Salles: Abbvie, Epizyme, Morphosys, Regeneron: Consultancy, Honoraria; Bayer: Honoraria; Beigene, BMS/Celgene, Debiopharm, Genentech/Roche, Genmab, Incyte, Ipsen, anssen, Novartis. Kite/Gilead, Loxo, Miltneiy, Rapt, TAKEDA, Velosbio, Allogene: Consultancy. Schuster: Acerta Pharma/AstraZeneca: Consultancy; Alimera Sciences: Consultancy; BeiGene: Consultancy; Juno Theraputics: Consultancy, Research Funding; Loxo Oncology: Consultancy; Tessa Theraputics: Consultancy; Genentech/Roche: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Research Funding; Adaptive Biotechnologies: Research Funding; Incyte: Research Funding; TG Theraputics: Research Funding; Novartis: Consultancy, Honoraria, Patents & Royalties, Research Funding; Abbvie: Consultancy, Research Funding; Nordic Nanovector: Consultancy; Celgene: Consultancy, Honoraria, Research Funding. Dreyling: BeiGene: Consultancy; Celgene: Consultancy, Research Funding, Speakers Bureau; Amgen: Speakers Bureau; Genmab: Consultancy; Gilead/Kite: Consultancy, Research Funding, Speakers Bureau; Incyte: Consultancy, Speakers Bureau; Janssen: Consultancy, Research Funding, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Abbvie: Research Funding; Astra Zeneca: Consultancy, Speakers Bureau; Bayer HealthCare Pharmaceuticals: Consultancy, Research Funding, Speakers Bureau. Kuruvilla: AbbVie: Honoraria; Amgen: Honoraria; AstraZeneca: Honoraria, Research Funding; Antengene: Honoraria; Roche: Honoraria, Research Funding; Incyte: Honoraria; BMS: Honoraria; Merck: Honoraria; Janssen: Honoraria, Research Funding; Karyopharm: Honoraria, Other: Data and Safety Monitoring Board; Novartis: Honoraria; Medison Ventures: Honoraria; Pfizer: Honoraria; Gilead: Honoraria; Seattle Genetics: Honoraria; TG Therapeutics: Honoraria. Patten: ROCHE: Research Funding; GILEAD SCIENCES: Honoraria, Research Funding; ABBVIE: Honoraria; NOVARTIS: Honoraria; ASTRA ZENECA: Honoraria; JANSSEN: Honoraria. Von Tresckow: AbbVie: Other: Congress and travel support; Amgen: Consultancy, Honoraria; AstraZeneca: Honoraria, Other: Congress and travel support; BMS-Celgene: Consultancy, Honoraria, Other: Congress and travel support; Kite-Gilead: Consultancy, Honoraria; MSD: Consultancy, Honoraria, Other: Congress and travel support, Research Funding; Novartis: Consultancy, Honoraria, Other: Congress and travel support, Research Funding; Pentixafarm: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Roche: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Other, Research Funding. Smith: Alexion, AstraZeneca Rare Disease: Other: Study investigator; Celgene, Genetech, AbbVie: Consultancy. Davis: Novartis, Vertex Pharmaceuticals, Pfizer, Eisai, Eli Lilly, AstraZeneca: Research Funding. Anjos: Novartis: Current Employment. Chu: Novartis: Current Employment, Current equity holder in publicly-traded company. Zhang: Novartis: Current Employment, Current equity holder in publicly-traded company. Lobetti Bodoni: Spouse: Harlcok Healthcare: Current holder of individual stocks in a privately-held company; Spouse: Takeda: Consultancy, Honoraria, Speakers Bureau; Spouse: Celgene: Honoraria; Spouse: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Spouse: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Spouse: Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Spouse: NHS: Ended employment in the past 24 months; Spouse: F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company; Gilead: Other: Travel sponsorship in June 2019; Novartis: Current Employment, Current equity holder in publicly-traded company. Thieblemont: Kyte: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Gilead Sciences: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses , Research Funding; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Bristol Myers Squibb/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Cellectis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses ; Hospira: Research Funding; Bayer: Honoraria; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Accommodations, Expenses . Fowler: BostonGene, Corp: Current Employment, Current holder of stock options in a privately-held company; Bristol Myers Squibb, F. Hoffmann-La Roche Ltd, TG Therapeutics and Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding. Dickinson: Janssen: Consultancy, Honoraria; Takeda: Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; MSD: Consultancy, Honoraria, Research Funding, Speakers Bureau; Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; Amgen: Honoraria; Celgene: Research Funding; Roche: Consultancy, Honoraria, Other: travel, accommodation, expenses, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Martinez-Lopez: Novartis: Consultancy, Speakers Bureau; BMS: Consultancy, Research Funding, Speakers Bureau; Janssen: Consultancy, Speakers Bureau; Incyte: Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Research Funding, Speakers Bureau; Astellas: Research Funding, Speakers Bureau. Wang: Eli Lilly: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; InnoCare: Research Funding; LOXO Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Research Funding; MorphoSys: Research Funding; TG Therapeutics: Membership on an entity's Board of Directors or advisory committees. Link: Genentech/Roche: Consultancy, Research Funding; MEI: Consultancy; Novartis, Jannsen: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal